Abstract
Introduction: CD19-directed autologous CAR-T products induce high response rates in adults with R/R B-ALL, yet many patients relapse within the first year. Additionally, cell manufacturing timelines, and poor t-cell fitness may imperil efficacy, especially among those with proliferative disease. This makes access to a donor-derived, readily available CAR-T product of great interest in this patient population, particularly when consolidation with allogeneic stem cell transplant (allo-SCT) is possible. We report preliminary safety, efficacy, and correlative data for the R/R B-ALL patients dosed with at least 3 x 10 6 CAR-T cells/kg of PBCAR0191, an allogeneic 'off-the-shelf' CD19-directed CAR-T.
Methods: Subjects were 18 years or older with CD19+ R/R B-ALL after at least 2 prior lines of therapy. Patients were required to have adequate organ function and no active GvHD, CNS disease, active infections, or other active medical issues. Prior allo-SCT and/or autologous CAR-T therapy were allowed. Subjects received either standard (sLD; 30mg/m2/day and 500mg/m2/day x 3 days fludarabine and cyclophosphamide, respectively) or enhanced (eLD; 30mg/m2/day x 4 days flu and 1000mg/m2/day x 3 days cy) lymphodepletion preceding PBCAR0191 infusion. Correlative laboratory samples were taken for CAR-T expansion, persistence, molecular response to treatment and safety assessments.
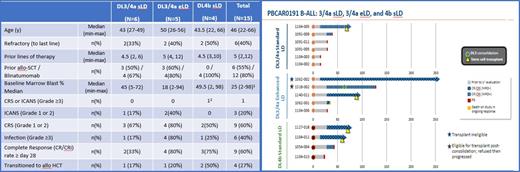
Results: As of August 2, 2021, 15 subjects with R/R CD19+ B-ALL have been dosed with dose Dose level 3/4a (3 X 10 6 CAR-T cells/kg or equivalent, n=11) or a Dose level 4b (flat dose of 5 X 10 8 CAR-T cells, n=4). Demographics, baseline disease, and prior treatment data are presented in the table. Most of the Adverse events (AE) reported to date were mild, with no cases of GvHD, no Grade ≥3 CRS and 1 case of Grade 3 ICANS which resolved within 48 hours. 67% of subjects treated (10/15) experienced PBCAR0191 related AEs, with 60% (9/15) of subjects experiencing serious AEs (one related to PBCAR0191, ICANS Grade 3).
The complete response (CR) or CRi (incomplete marrow recovery) rate at Day ≥28 is 33% (2/6) in DL3/4a and sLD, 80% (4/5) in DL3/4a with eLD and 75% (3/4) in DL4b with sLD. Importantly, 4/15 (27%) responding subjects underwent allo-SCT, with one additional subject not able to receive transplant due to eligibility yet maintaining an MRD- CR for >250 days, and one refusing to proceed with transplant. Product accessibility was evident compared to autologous CAR-T products, with median time from screening completion to PBCAR0191 infusion of 7 days (median of 1 day until start of LD) and all eligible subjects receiving PBCAR0191 infusion.
Conclusion: PBCAR0191 has demonstrated a manageable safety profile and high complete response rate at day 28 or later, providing an adequate window for potential bridge to allo-SCT.
Jain: Adaptive Biotechnologies: Honoraria, Research Funding; Precision Biosciences: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; Pfizer: Research Funding; Janssen: Honoraria; Genentech: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Beigene: Honoraria; TG Therapeutics: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Aprea Therapeutics: Research Funding; Incyte: Research Funding; AbbVie: Honoraria, Research Funding; Fate Therapeutics: Research Funding; Servier: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Pharmacyclics: Research Funding. Kantarjian: KAHR Medical Ltd: Honoraria; Ascentage: Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Aptitude Health: Honoraria; Ipsen Pharmaceuticals: Honoraria; Precision Biosciences: Honoraria; Novartis: Honoraria, Research Funding; Astra Zeneca: Honoraria; AbbVie: Honoraria, Research Funding; NOVA Research: Honoraria; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Astellas Health: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Sauter: Bristol-Myers Squibb: Research Funding; GSK: Consultancy; Celgene: Consultancy, Research Funding; Gamida Cell: Consultancy; Kite/Gilead: Consultancy; Precision Biosciences: Consultancy; Genmab: Consultancy; Novartis: Consultancy; Spectrum Pharmaceuticals: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Sanofi-Genzyme: Consultancy, Research Funding. Heery: Precision BioSciences: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Arcellx: Current Employment, Current holder of stock options in a privately-held company. List: Halia Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company; CTI Biosciences: Consultancy; Precision BioSciences: Current Employment, Current equity holder in publicly-traded company; Aileron Therapeutics: Consultancy. Johnson: Precision BioSciences, Inc: Current Employment, Current equity holder in publicly-traded company. Lou: Precision BioSciences: Current Employment, Current equity holder in publicly-traded company. Vainorius: Precision BioSciences: Current Employment, Current equity holder in publicly-traded company; Abbvie: Current equity holder in publicly-traded company; United Therapeautics: Current equity holder in publicly-traded company. Olszewski: Genentech, Inc.: Research Funding; TG Therapeutics: Research Funding; PrecisionBio: Research Funding; Celldex Therapeutics: Research Funding; Acrotech Pharma: Research Funding; Genmab: Research Funding. Stein: Amgen: Consultancy, Speakers Bureau; Celgene: Speakers Bureau; Stemline: Speakers Bureau. Shah: Adaptive Biotechnologies: Consultancy; Bristol-Myers Squibb/Celgene: Consultancy, Other: Expenses; Novartis: Consultancy, Other: Expenses; Pfizer: Consultancy, Other: Expenses; Amgen: Consultancy; Precision Biosciences: Consultancy; Kite, a Gilead Company: Consultancy, Honoraria, Other: Expenses, Research Funding; Pharmacyclics/Janssen: Honoraria, Other: Expenses; Acrotech/Spectrum: Honoraria; BeiGene: Consultancy, Honoraria; Incyte: Research Funding; Jazz Pharmaceuticals: Research Funding; Servier Genetics: Other.
PBCAR0191 is not FDA approved

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal